Shares of Merus N.V. MRUS are trading higher Tuesday after the U.S. Food and Drug Administration (FDA) granted Breakthrough Therapy designation (BTD) to petosemtamab in combination with pembrolizumab.
What To Know: The designation applies to the first-line treatment of adult patients with recurrent or metastatic PD-L1 positive head and neck squamous cell carcinoma (r/m HNSCC) with a combined positive score of at least 1.
This marks the second BTD for petosemtamab, following an earlier designation for patients with r/m HNSCC whose disease had progressed after treatment with platinum-based chemotherapy and an anti-PD-1 antibody. The new designation is based on updated data from an ongoing phase 1/2 clinical trial, which initially showed a 67% response rate among 24 evaluable patients.
Breakthrough Therapy designation is intended to expedite the development and review of drugs that show substantial improvement over existing treatments for serious conditions. This status will allow Merus to work closely with the FDA, accelerating regulatory discussions and potentially leading to a faster approval process.
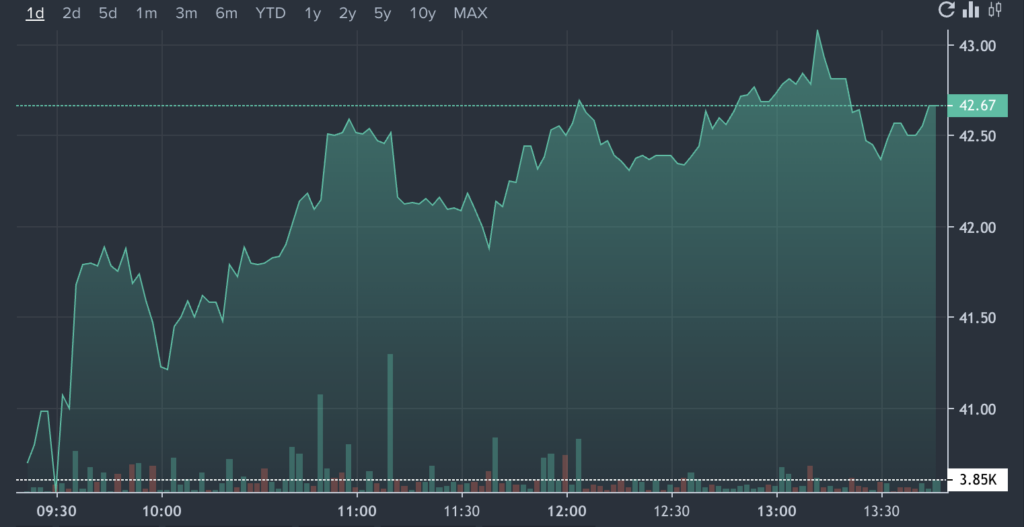
MRUS Price Action: Merus shares were up 5.40% at $42.37 at the time of writing, according to Benzinga pro.
Read Next:
Image via Shutterstock.
© 2025 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.