TransCode Therapeutics Inc. RNAZ shares are moving higher Friday after the company announced that the first patient in Cohort 3 of its Phase 1 clinical trial has been dosed with its lead candidate, TTX-MC138 on Tuesday.
What To Know: The company’s Safety Review Committee approved the progression to the third cohort following a favorable review of safety and pharmacokinetic data from Cohorts 1 and 2. No significant safety or dose-limiting toxicities have been reported in the earlier cohorts. The pharmacokinetic data from these cohorts has been consistent with preclinical and Phase 0 trial results. Patients from the earlier cohorts remain in the study and are continuing to receive additional doses of TTX-MC138.
The third cohort will receive a dose approximately double that administered in the second cohort. Preliminary analyses indicate that TTX-MC138 has demonstrated pharmacodynamic activity, with a 66% inhibition of miR-10b expression at 24 hours post-infusion, similar to findings from the earlier Phase 0 trial. The increase in activity observed with the escalated dose in Cohort 2 suggests a favorable pharmacokinetic profile for the drug.
Sue Duggan, TransCode’s Senior Vice President of Operations, commented that the commitment from clinical sites could enable a swift completion of the third cohort. Enrollment in the study is ongoing based on the Safety Review Committee’s continued review of cumulative safety data.
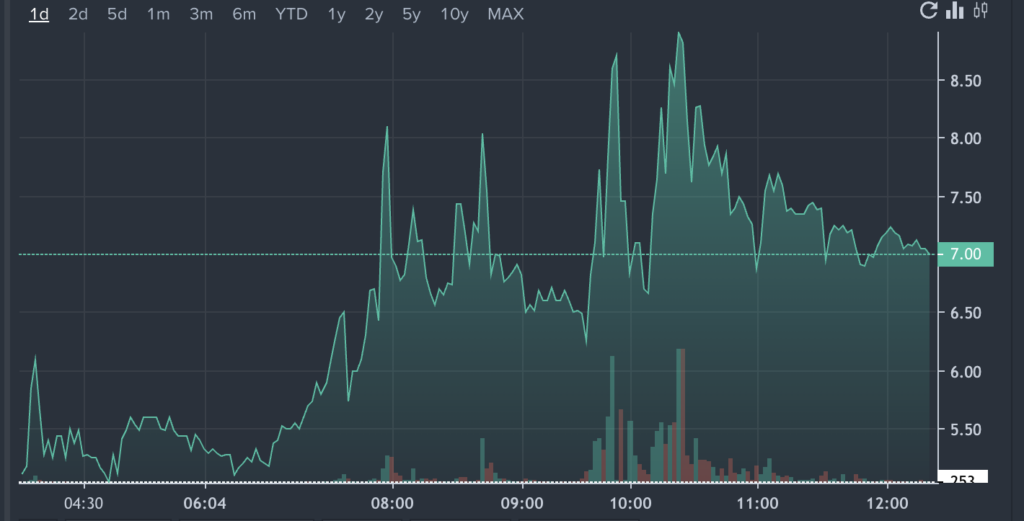
RNAZ Price Action: TransCode shares were up 21.2% at $6.67 at the time of writing, according to Benzinga Pro.
Read Next:
Image via Transcodetherapeutics.
Don't miss a beat on the share market. Get real-time updates on top stock movers and trading ideas on Benzinga India Telegram channel.
© 2025 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.